
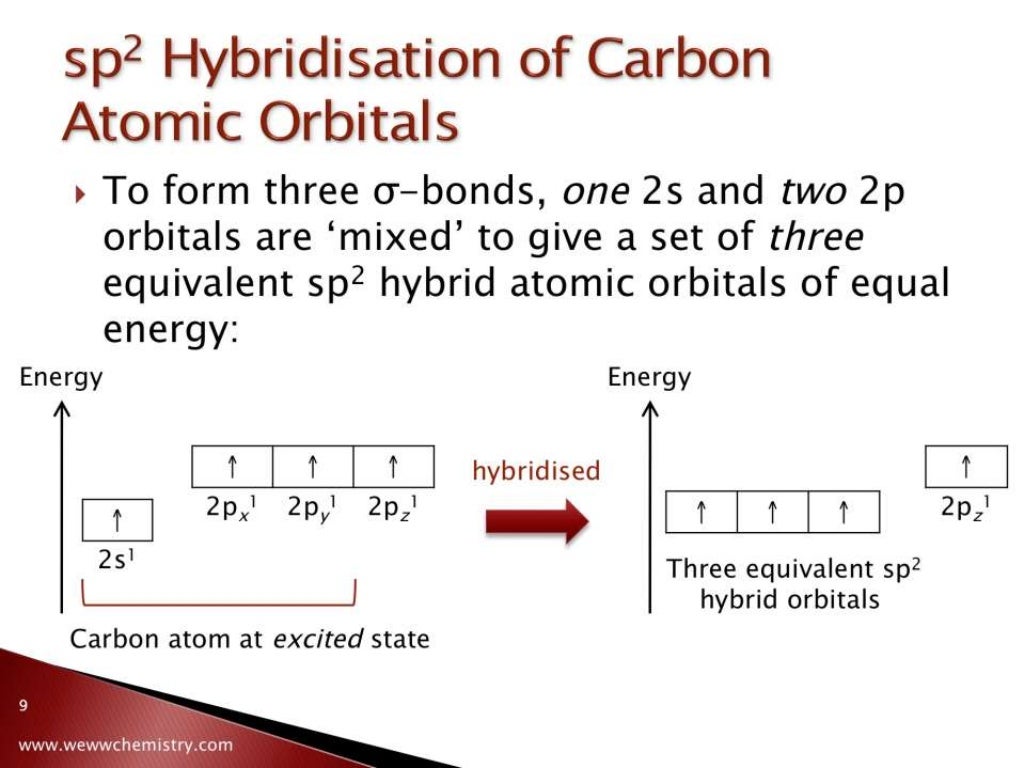
Remember, the steric number is the sum of the atoms and lone pairs on an atom, and it dictates the hybridization and the geometry of that atom. The central atom has 3 atoms and a lone pair, so the steric number is equal to 4 (SN = 4). This one hybrid orbital accommodates the lone pair of the nitrogen, while the other three overlap with the s orbitals of hydrogen thus making three sigma N-H bonds. Now, the principle is the same as we have just seen for carbon, however, when the three p orbitals are mixed with one s orbital, four sp 3 orbitals are formed where one of them is filled with two electrons: Nitrogen is the next element after carbon, with an electron configuration of 1s 22s 22p 3. Let’s consider an example where the atom is sp 3-hybridized but instead of four atoms, it has three atoms and a lone pair. And the way to look at this is, in order for the four groups to be as far away from each other as possible like we learned in the VSEPR theory, the groups need to be in identical four orbitals which is only possible in the sp 3 hybridization.
To generalize this, any atom with four groups (either an atom or a lone pair) is sp 3 hybridized. If instead of one hydrogen, we connect another sp3-hybridized carbon, we will get ethane:Īnd consequently, in all the alkanes, there is a sigma bond between the carbon atoms and the carbon-hydrogen atoms, and the carbons are sp 3 hybridized with tetrahedral geometry: The bonds that form by the head-on overlap of orbitals are called σ (sigma) bonds because the electron density is concentrated on the axis connecting the C and H atoms. The four sp 3-hybridized orbitals arrange in a tetrahedral geometry and make bonds by overlapping with the s orbitals of four hydrogens: This explains the symmetrical geometry of methane (CH 4) where all the bonds have the same length and bond angle.Īll four C – H bonds in methane are single bonds that are formed by head-on (or end-on) overlapping of sp 3 orbitals of the carbon and s orbital of each hydrogen. The formation of these degenerate hybrid orbitals compensates for the energy uphill of the s-p transition as they have lower energy than the p orbitals. And again, we call them sp 3 because they are formed from one s orbital and three p orbitals. These are hybrid orbitals and look somewhat like the s and p orbitals. The number of the hybrid orbitals is always the same as the number of orbitals that are mixed. So, four orbitals (one 2s + three 2p) are mixed and the result is four sp 3orbitals. So, in the next step, the s and p orbitals of the excited state carbon are hybridized to form four identical in size, shape, and energy orbitals. Pay attention that the electron goes uphill as the p subshell is higher in energy than the s subshell and this is not energetically favorable, but we will see how it is compensated in the next step when orbitals are mixed (hybridized). This leads to the excited state of the carbon: In the first step, one electron jumps from the 2s to the 2p orbital. Now, let’s see how that happens by looking at methane, CH 4 as an example. Hybridization is a theory that is used to explain certain molecular geometries that would have not been possible otherwise. And this is where we get into the need of a theory that can help us explain the known geometry and valency of the carbon atom in many organic molecules. You can see from the electron configuration that it is impossible to make four, identical in bond length, energy, and everything else (degenerate) bonds because one of the orbitals is a spherical s, and the other three are p orbitals. The valence electrons are the ones in the 2s and 2p orbitals and these are the ones that participate in bonding and chemical reactions. So, in order to predict the valency and geometry of the carbon atom, we are going to look at its electron configuration and orbitals. Remember also that covalent bonds form as a result of orbital overlapping and sharing two electrons between the atoms. A reminder that in tetrahedral geometry, all the angles are 109.5 o and the bonds have identical lengths. It is confirmed experimentally that the carbon atom in methane (CH 4) and other alkanes has a tetrahedral geometry. Let’s start first by answering this question: Why do we need the hybridization theory?


 0 kommentar(er)
0 kommentar(er)
